Learning Outcomes
By the end of this lesson, students should be able to:
i. Explain the concept of enzyme activity and its significance in biological processes.
ii. Identify temperature and pH as crucial factors that affect enzyme activity.
iii. Describe the effect of temperature on enzyme activity, including the concept of optimal temperature range and denaturation.
iv. Explain the impact of pH on enzyme activity, relating it to the protonation state of amino acids in the enzyme's active site.
v. Appreciate the importance of considering environmental factors when predicting enzyme efficiency in various biological contexts.
Introduction
Enzymes, the master catalysts of life, play an indispensable role in accelerating biochemical reactions that are essential for various biological processes. However, their activity is not constant and can be influenced by environmental factors, such as temperature and pH. Understanding the impact of these factors on enzyme activity is crucial for predicting their efficiency in various biological contexts.
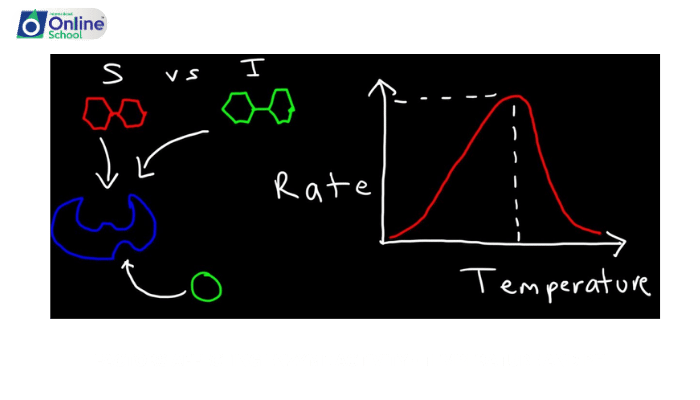
i. Temperature: A Crucial Regulator of Enzyme Activity
Temperature plays a significant role in determining enzyme activity. Each enzyme has an optimal temperature range, within which its activity is highest. As temperature increases within this range, the kinetic energy of the molecules increases, leading to more frequent collisions between enzymes and their substrates. This results in increased reaction rates and enzyme activity.
ii. Denaturation: The Unfolding of Enzyme Structure
Exceeding the optimal temperature range can lead to enzyme denaturation, a process where the enzyme's three-dimensional structure is disrupted, rendering it inactive. Denaturation occurs because the increased thermal energy causes the non-covalent bonds that maintain the enzyme's structure to break, leading to a loss of the active site's shape and inability to bind substrates.
iii. pH: Influencing the Active Site's Chemistry
The pH of the surrounding environment also significantly impacts enzyme activity. Enzymes have an optimal pH range, where the protonation state of amino acids in the enzyme's active site is suitable for catalysis. Deviations from this optimal pH can alter the active site's chemistry, preventing substrate binding or interfering with the catalytic process.
iv. Consequences of pH Disruptions on Enzyme Activity
Extreme pH conditions can lead to irreversible denaturation of enzymes. In acidic environments, excess protons can protonate amino acids, altering their charge and disrupting the enzyme's structure. In alkaline conditions, hydroxide ions can deprotonate amino acids, again affecting the enzyme's structure and catalytic ability.
v. Predicting Enzyme Efficiency in Diverse Environments
Understanding the influence of temperature and pH on enzyme activity is essential for predicting their efficiency in various biological contexts. For instance, enzymes involved in digestion must function within the pH range of the digestive tract, while enzymes in thermophilic organisms, such as bacteria living in hot springs, must be stable at high temperatures.
Temperature and pH are crucial factors that modulate enzyme activity, influencing their ability to accelerate biochemical reactions. Understanding the impact of these environmental conditions is essential for appreciating the intricate workings of enzymes in various biological processes, from digestion and energy production to disease development and drug action.